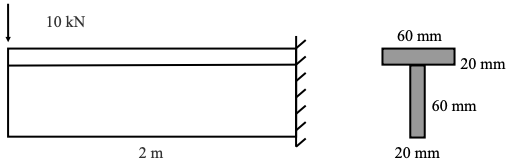
Explanation:
The second moment of area of the cross-section is 1.36 × 10−6𝑚𝑚4
The top of the beam is in tension.
The centroid is 50 mm up from the bottom of the cross-section.
The maximum moment is 20 KN–m.
Explanation:
A normal compressive stress in the y-direction of 60 MPa is a negative stress.
Explanation:
Mostly this requires the student to remember that adiabatic means (by definition) that there is no flow of heat. Then one must make sure that (B) and (C) do not also generally accompany an adiabatic process. While an adiabatic process may have no temperature change or enthalpy change, this is not generally true. For instance, enthalpy and temperature both change for an adiabatic turbine.
Explanation:
It's calculated using the formula for the coefficient of performance (COP) of a Carnot refrigerator.
Explanation:
The final pressure is approximately 2.50 bars.
Explanation:
The outlet temperature is unknown. The procedure is to solve for 𝑊𝑊/𝑚𝑚 for the ideal case (where the outlet temperature can be calculated) and then apply the efficiency to get the actual 𝑊𝑊/𝑚𝑚. The ideal case is reversible and is also adiabatic. An adiabatic and reversible process is isentropic. For an isentropic process involving an ideal gas with constant heat capacity.
The actual compressor requires more energy and is obtained from
W/m = (W/m)ideal/efficiency
= 174/0.7
= 249kJ/kg
Explanation:
Hoop stress in the tank governs the maximum allowable stress.
Hoop stress is equal to circumferential stress and tangential stress.
Advertisement
Explanation:
Heat rejection in a Carnot cycle occurs during the isothermal compression phase. During this phase, the working substance releases heat to the surroundings at a constant temperature.
Explanation:
The purpose of a condenser in a Rankine cycle is to convert the working fluid from vapor to liquid by removing heat from it. This process occurs at constant pressure.
Explanation:
Entropy remains constant during an isentropic process. This implies that there is no change in the degree of disorder of the substance.
Explanation:
The entropy of a closed system increases in an irreversible process. This is because irreversible processes involve an increase in disorder or randomness within the system.