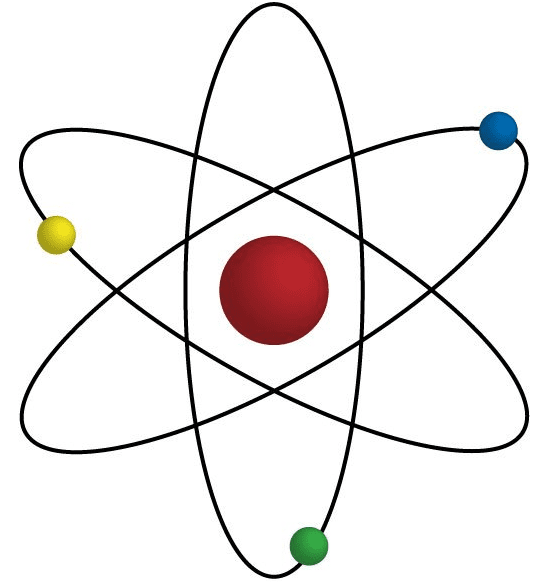
These three particles - protons, neutrons, and electrons - together form the basic structure of atoms. Protons and neutrons are located in the nucleus, which contains most of the atom's mass and is positively charged due to the presence of protons.
Electrons occupy the space surrounding the nucleus and contribute to the atom's size and chemical behavior. The number of protons in an atom determines its atomic number (Z), which defines the element, while the combined number of protons and neutrons determines the atomic mass number (A).
"Magnesium hydroxide is a compound composed of magnesium (Mg) and hydroxide (OH) ions. In this compound, magnesium (Mg) has a +2 charge, and the hydroxide (OH) ion has a -1 charge.
The formula Mg(OH)2 indicates that magnesium (Mg) is bonded to two hydroxide (OH) ions. This chemical formula correctly represents the composition and structure of magnesium hydroxide. Each magnesium ion (Mg^2+) is surrounded by two hydroxide ions (OH^-) in the compound. Therefore, the proper formula for magnesium hydroxide is Mg(OH)2."
The three types of nuclear radiation in order of increasing penetrating power are alpha (α) particles, beta (β) particles, and gamma (γ) rays.
1.Alpha (α) particles: Alpha particles consist of two protons and two neutrons bound together, similar to the nucleus of a helium atom. They have a relatively low penetrating power and can be stopped by a sheet of paper or a few centimeters of air. However, alpha particles can be harmful if ingested or inhaled.
2.Beta (β) particles: Beta particles are high-energy electrons (β^-) or positrons (β^+). They have greater penetrating power compared to alpha particles and can penetrate several millimeters of materials like aluminum. Beta particles can be shielded by thicker materials such as plastic or glass.
3.Gamma (γ) rays: Gamma rays are high-energy electromagnetic waves emitted from the nucleus of an atom during radioactive decay.
They have the highest penetrating power and can pass through several centimeters of dense materials like lead or concrete. Specialized shielding such as lead or concrete is required to protect against gamma rays.
Therefore, the correct order of increasing penetrating power for the three types of nuclear radiation is alpha (α), beta (β), and gamma (γ), represented by option (a) alpha, beta, gamma.
The chemical formula C6H12O6 represents glucose, which is a type of sugar and an organic compound. This formula indicates the composition of glucose in terms of its constituent elements:
1.C6 (carbon): There are 6 carbon atoms in each molecule of glucose.
2.H12 (hydrogen): There are 12 hydrogen atoms in each molecule of glucose.
3.O6 (oxygen): There are 6 oxygen atoms in each molecule of glucose.
Therefore, the compound C6H12O6 consists of 3 elements: carbon (C), hydrogen (H), and oxygen (O). Each element contributes to the molecular structure of glucose, which is essential for energy production in living organisms through cellular respiration.
A molecule is a group of two or more atoms that are chemically bonded together. These atoms can be of the same element (forming a molecule of an element) or different elements (forming a molecule of a compound). In a molecule, atoms are held together by chemical bonds, such as covalent bonds, where atoms share electrons to achieve stability.
Isomers are molecules that have the same molecular formula (same number and types of atoms) but different structural formulas (different arrangement of atoms).
Isomers can exhibit differences in the connectivity of atoms or spatial arrangement, leading to distinct chemical and physical properties
Alkanes are a group of hydrocarbons that consist of single bonds between carbon atoms.
They are also known as saturated hydrocarbons because each carbon atom in an alkane is bonded to four other atoms (hydrogen atoms), resulting in the maximum number of hydrogen atoms per carbon atom.
The general formula for alkanes is CnH2n+2, where ""n"" represents the number of carbon atoms.
Alkanes have straight-chain or branched-chain structures and are characterized by their stability and lack of reactivity due to the presence of single bonds.
Advertisement
These fats contain at least one double bond in their hydrocarbon chain, but not all molecules with double/triple bonds are fats.
The chemical formula C4H10 corresponds to butane, which is an alkane hydrocarbon. Butane is a saturated hydrocarbon composed of four carbon atoms and ten hydrogen atoms.
It belongs to the alkane family and is characterized by single bonds between carbon atoms.
The group made up of alcohols and acids is called esters. Esters are organic compounds that are formed by the reaction between an alcohol (containing an -OH group) and a carboxylic acid (containing a -COOH group).
This reaction is known as esterification.
In ester molecules, the -OH group of the alcohol and the -COOH group of the acid combine to form an ester linkage (-COO-) along with the elimination of a water molecule (H2O). Esters have a general formula RCOOR', where R and R' are alkyl or aryl groups.
Esters are widely used in various applications, including as flavorings and fragrances in food and perfumes, as solvents in industry, and as plasticizers in polymers.
Hydrocarbons are compounds composed solely of carbon (C) and hydrogen (H) atoms.