FREE EIT Thermodynamics and Strength of Materials Questions and Answers
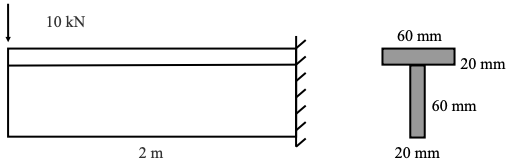
A cantilever beam is loaded with a force of 10 KN and has a cross-section as shown. The maximum tensile stress in MPa in the beam most nearly is:
Explanation:
The second moment of area of the cross-section is 1.36 × 10−6𝑚𝑚4
The top of the beam is in tension.
The centroid is 50 mm up from the bottom of the cross-section.
The maximum moment is 20 KN–m.
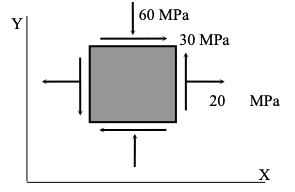
The maximum tensile principal stress in MPa for the two-dimensional stress state given is:
Explanation:
A normal compressive stress in the y-direction of 60 MPa is a negative stress.
In all adiabatic processes, there is:
Explanation:
Mostly this requires the student to remember that adiabatic means (by definition) that there is no flow of heat. Then one must make sure that (B) and (C) do not also generally accompany an adiabatic process. While an adiabatic process may have no temperature change or enthalpy change, this is not generally true. For instance, enthalpy and temperature both change for an adiabatic turbine.
A Carnot refrigerator operates between 10°𝐶𝐶 and 25°𝐶𝐶. The coefficient of performance is:
Explanation:
It's calculated using the formula for the coefficient of performance (COP) of a Carnot refrigerator.
Air in a closed-rigid vessel at 2 bars and 25°𝐶𝐶 is heated until its temperature is 100°𝐶𝐶. The final pressure in bars is:
Explanation:
The final pressure is approximately 2.50 bars.
Air is adiabatically compressed in a steady flow system from 1 bar to 5 bars. The air enters at 298 K and the compressor efficiency is 70 %. The work per unit mass of air required for the compression (in kJ/kg) is:
Explanation:
The outlet temperature is unknown. The procedure is to solve for 𝑊𝑊/𝑚𝑚 for the ideal case (where the outlet temperature can be calculated) and then apply the efficiency to get the actual 𝑊𝑊/𝑚𝑚. The ideal case is reversible and is also adiabatic. An adiabatic and reversible process is isentropic. For an isentropic process involving an ideal gas with constant heat capacity.
The actual compressor requires more energy and is obtained from
W/m = (W/m)ideal/efficiency
= 174/0.7
= 249kJ/kg
A 1-meter diameter steel cylindrical tank is used to store propane at high pressure. If the allowable stress of steel is 100 MPa and the propane is stored at a pressure of 1000 kPa, the minimum thickness in mm of the tank is:
Explanation:
Hoop stress in the tank governs the maximum allowable stress.
Hoop stress is equal to circumferential stress and tangential stress.
Advertisement
At what point in a Carnot cycle does heat rejection occur?
Explanation:
Heat rejection in a Carnot cycle occurs during the isothermal compression phase. During this phase, the working substance releases heat to the surroundings at a constant temperature.
What is the purpose of a condenser in a Rankine cycle?
Explanation:
The purpose of a condenser in a Rankine cycle is to convert the working fluid from vapor to liquid by removing heat from it. This process occurs at constant pressure.
Which property of a substance remains constant during an isentropic process?
Explanation:
Entropy remains constant during an isentropic process. This implies that there is no change in the degree of disorder of the substance.
What happens to the entropy of a closed system in an irreversible process?
Explanation:
The entropy of a closed system increases in an irreversible process. This is because irreversible processes involve an increase in disorder or randomness within the system.