MCAT (Chemical and Physical Foundations)
While investigating mining samples off the coast of Columbia, a scientist discovers an element that has never been detected by humans. The molecule possesses an electronegativity similar to iron, according to extensive testing (Fe). What would the following values be regarding magnesium if the characteristics of this element were found to be consistent with iron? Make use of the periodic table as a guide.
Answer Explanation: (C)
This question necessitates knowledge of trends along the periodic table. Ionization energy and
electronegativity increase in the same direction, up and to the right. Atomic radius, however, moves in the opposite direction.
These three trends will allow arrival to the correct answer.
How many grams of carbon dioxide would arise from a reaction with 84 grams of ethane and infinite oxygen
(Carbon atomic weight: 12amu, Hydrogen atomic weight: 1amu, Oxygen atomic weight: 16amu) using this formula?
The following is the imbalanced ethane gas reaction with carbon dioxide and water:
CO2 + H2O (C2H4 + O2)
Answer Explanation: (D)
This question first dictates that the equation must be balanced. The stem will not always indicate that the equation is unbalanced,
so this must be affirmed before proceeding. The balanced equation is as follows:
C2H4 + 3O2 —> 2CO2 + 2H2O
Next, using the given amount of reactant, you should use the atomic weights to calculate the moles. 1 mole of ethane is equal to
28 amu (24+4) or grams. Thus, if provided with 84g of ethane, there will be 3 moles. Since there is a 1:2 conversion of ethane to CO2,
this will result in 6 moles of carbon dioxide. Using atomic weight to calculate, 6 moles x 44amu = 264g of CO2.
Tip: Practice these equations with simple numbers, since the concept is the same.
The equation below explains how ammonia is produced:
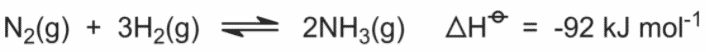
Answer Explanation: (E)
This question tests Le Chatelier’s principle. There are several key components to this principle, all in regards
to steady state equations. Increasing the substance will shift the equation away from that substance in order to use up extra concentration.
Increasing the pressure of the system will push the equation in the direction that has fewer moles of gas, as the volume decreases. Increasing
the temperature will favor an exothermic reaction. Using this information, it is possible to derive the answer. Important note: adding a catalyst
will not shift the equation, as the rates of both forward and backward will be increased.
On a flat track with a ramp at the finish, a race car attempts to leap a string of eight buses. Engineers working on the project concluded that the car needed achieve a speed of 130 km/h in order to leap the buses. What rate must the car accelerate to obtain this velocity if the track distance is 50m?
Answer Explanation: (B)
This is a fairly straightforward problem assessing the knowledge of kinematic equations. The variables given are distance (50m), start velocity
(0 km/h), and final velocity (130 km/h). Converting the velocity into meters per second will yield 36 m/s. Since acceleration is assumed to be
constant, averaging the start and final velocities will yield the average velocity (18 m/s). The change in velocity (36 m/s) over the change in time
is equal to the acceleration of the car. Thus, if rearranged, this means that the change in time from point A to point B is equal to the change in
velocity divided by acceleration. One of the displacement equations is as follows:
S = (Vf - Vi)/2 x (∆t)
Thus, using the change in velocity over the change in acceleration as ∆t, and using displacement as the total distance of 50m, the answer can
be derived as 12.96 m/s2, which can be rounded to 13 using significant figures.
A nozzle is always attached to the end of a fire hose, which works in part by reducing the area of water exiting the fire hydrant to create a more powerful stream. What is the final velocity of water as it exits a fire hydrant if the initial velocity is 2 m/s, pressure is held constant, and the end of the nozzle is 1/3 the area of the start of the hose?
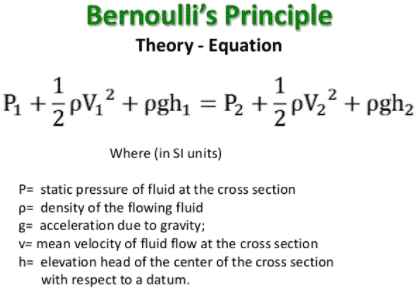
Answer Explanation: (A)
Bernoulli’s Principle describes the conservation of energy within a fluid. The entire Bernoulli equation is listed above:
However, in cases such as this, where both the heights, densities, are the same, the more simplified conservation of energy question
can be used, which is:
A1V1 = A2V2
Thus, if the areas and one of the starting volumes are known, this turns into a very easy question. Simply plugging the values to this
arrives at the correct answer. We know that A2 is 1/3 that of A1, and we know the velocity of V1. Using these numbers, the correct
answer becomes a corresponding increase in velocity.
For this chemical, which of the following would be the correct nomenclature?
Under spectroscopy, a laboratory-made chemical was discovered to contain the following structure:
Answer Explanation: (D)
Nomenclature in organic chemistry follows specific rules. First, identify the longest carbon chain, which in this case is an 8-carbon line.
Next, identify any double bonds, as this will change the name. Since there are none, this is an alkane. This immediately narrows it down
to 2 answer choices. Next, the name should have the lowest cumulative number possible. This rules out answer A.
How would this compound be labeled correctly using nomenclature rules?
After successfully synthesizing their drug, the same scientist employed a more sophisticated process that used bromine.
She was left with the following structure after the response was finished:
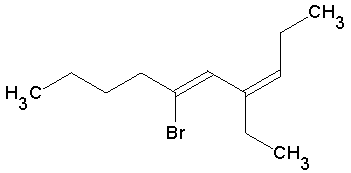
Answer Explanation: (B)
The recognition of double bonds, if done correctly, can help speed the process of naming compounds. Since this compound has double
bonds, the numbering system starts closest to those bonds. Although this does not change the numbering anyway, it is an easy way for
test makers to assess this knowledge. Next, the compound name is done alphabetically. Finally, the double bonds are named by the first
carbon of their double bond. This will arrive you at the correct answer.
In the laboratory, a transmembrane protein is discovered to be made up of four distinct amino acids in various amounts. Glycine, tyrosine, arginine, and isoleucine are the most common amino acids. Which amino acid is most likely to be found within the transmembrane domain?
Answer Explanation: (B)
Memorization of amino acids is an expected component of the MCAT. The start of this question rests on the soft fact that hydrophobic
acids will be found inside the lipid bilayer more often, which should be apparent.
Tip: Amino acids with lots of carbons are likely hydrophobic.
A new enzyme is found in a transgenic mice that participates in synthesis of an unknown product using two reactants. When using radiolabeled compounds to study the enzyme, it is found that the enzyme catalyzes a process that switches a nitrogen group on one reactant to the other reactant. Which of the following categories would this new enzyme fall under?
Answer Explanation: (A)
This question highlights the six classes of enzymes that exist according to their properties. In order, oxidoreductases transfer electrons
between reactants. Transferases move a functional group from one reactant to another. Hydrolases cleave a molecule using water to
produce two products. Lyases dissociate a molecule into two products without using water or oxidation/reduction. Isomerases convert
a reactant to its isomer. Ligases form a complex between reactants.
On the third day of an evolution symposium, two scientists take the stage to debate their beliefs. Each is a passionate follower of their respective philosophy. According to the first scientist, organisms evolved by increasing the number of organs that were employed the most at the time. They'd then pass them down to future generations. The second scientist, on the other hand, believed that each organism's advantages were absent for a long time, then appeared at random, and when they were helpful, that organism would swiftly populate the population over a short period of time, according to evolution. Which of the following statements would support the argument of the second scientist?
Answer Explanation: (D)
Scientist 2 ascribes to a evolutionary philosophy known as punctuated equilibrium, while Scientist 1 is more devout to acquired characteristics,
which was popularized by Lamarck. It is fairly easy to discern this from reading the stem. However, one of the ways this can be tested is that
Darwinism, or the Natural Selection Theory, is mostly correct, but has some modern advances that are considered different theories. Darwin
suggested these mutations happened steadily and over time, which is what would differ from the punctuated equilibrium theory.
A high school science teacher pours pure nitrogen into a 1 liter bottle and closes the lid. The room temperature is 25°C and the pressure is 1.70 atm. If all other factors remain constant, which two variables will both increase the system's pressure?
Answer Explanation: (C)
This question solicits knowledge of the ideal gas law, PV = nRT. There are several derivatives of this law that are helpful, but most information
can be solicited in these questions using this original form. Increasing temperature increases the speed at which molecules of the gas move
and thus increase pressure. Additionally, increasing the moles of gas will increase the amount of molecules in the system, and without a change
in volume, will increase pressure. Increasing the volume of the system allows for expansion of the gas and subsequent decrease in pressure.
Perchloric acid (HClO4) is one of the most powerful acids the world has ever known. Which of the following statements most closely describes strong acids?
Answer Explanation: (D)
The understanding of what makes an acid strong vs. weak is an important concept for the MCAT. Strong acids are such because their
conjugate bases are very stable (in many cases, such as perchloric acid, this is because their outer valence rings are filled), and thus in
the presence of an aqueous solution, such as water, they readily dissociate. A strong acid will have a Ka greater than 1, indicating that
it more readily dissociates.
R1 = 5, R2 = 10, R3 = 15, and V = 8.1V are the provided values for this circuit. Which of the following would cause the current to increase to 6.0A?
Given above is the diagram of a resistor circuit:
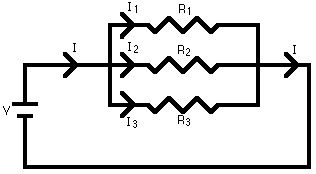
Answer Explanation: (B)
Resistor circuits are easy ways to get points on the MCAT. The first step in this problem is to determine the resistance of the circuit (Req).
The common denominator of the resistances is 30. Since 1/Req = 1/R1 + 1/R2 …., 1/Req = 11/30 = 2.7Ω. Second step is to determine the
current. Since V = IR, rearranged is I = V/R = 8.1/2.7 = 3.0A. Using this number, you can then plug in the theoretical solutions until you arrive
at the correct answer (Remember that, when adding in parallel, Req = R1 + R2 …). To check work, remember that resistors added in parallel
decrease the overall resistance, whereas resistors added in series always increase overall resistance.
A 1 meter tall jug of water springs a leak from a weak place in the plastic at the very bottom of the side, while sitting on a 2 meter high tabletop with its lid open. How quickly will the water in the jug empty?
Answer Explanation: (B)
This is another question testing the principles of Bernoulli’s equation. However, since height is not involved, the conservation of energy
equation is too simple. Several things in the equation can be simplified. Water has the same density, start velocity of standing water is zero,
and pressure is the same since both are exposed to normal atmospheric pressure. Thus, the rearranged equation reads:
(1/2)v22 = gh1 - gh2
Remember that, although a height for the countertop is given, it is irrelevant, since g remains the same. Thus h1 can be 1m, and h2 can be 0m,
roughly. Solving for this will give you the answer.
Tip: When possible, reduce out all stable variables. The equations will be much easier.
Which of the following statements about the procedure described here (compound-titrating solution) is correct?
In the lab, a titratable substance is submitted to titration. The graph below depicts the curve:
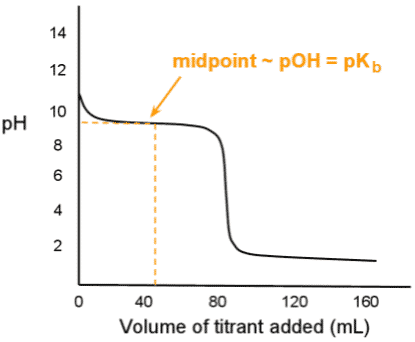
Answer Explanation: (C)
This question will help conceptually understand titration curves, with emphasis on the differentiating points that will determine the correct
answer. Any solution being titrated, whether an acid or a base, will show a steep initial change near the y-axis if it is weak. That, along with
the starting pH, gives you the starting substrate. Secondly, a rapid decline at the equivalence point (almost vertical) and a low final pH indicates
that the titrating substance in this example is a strong acid. These facts altogether should elicit the correct answer on most of these questions.
During DNA replication, around for per 100,000/1 million copies, an error is written into the leading strand. Several mechanisms are used to proofread this DNA. Which repair process is most likely at action if a mistake is discovered and the erroneous base is deleted immediately after the RNA primer is removed?
Answer Explanation: (C)
DNA repair is a crucial biological mechanism to ensure proper coding. The first line of defense against an improperly coded base is
3’-5’ activity by DNA polymerase III, which both synthesizes DNA and checks for mistakes in the reverse direction. Followed by this,
DNA polymerase I, while removing the RNA primer, checks for mistakes in the 5’-3’ direction. Finally, mismatch repair proteins check
synthesized DNA strands for mistakes. Endonuclease activity describes going inside a DNA strand to repair a mistake, whereas the
mistake described above was corrected by excising a single base before ligation.
A specific amount of 2-bromobutane is added to a strong ethanol solution and allowed to react completely. This reaction yields a major product of 2-butene and a minor product of 1-butene as a consequence. Which of the following statements about the beginning molecule best explains why 2-butene is the most important product?
Answer Explanation: (B)
This question assesses the test taker’s knowledge of Zaitsev’s Rule. The leading theory of this rule is that, in an E2 reaction of a strong base
and a compound with 2 distinct beta carbon groups, the reaction will favor towards double bonding of the beta carbon with less hydrogens.
It would be extremely helpful to visualize this on the test by writing it out if the starting compounds are simple, but if they are complex, knowing
the current reasoning for this rule is sufficient.
A sound technician is tasked with adjusting the frequency of a gunshot to more precisely match the typical speed of sound while working on a scene for an action film. The gunshot was fired by an actor inside a car going at 108 kilometers per hour, and it was captured by a camera on a platform 200 meters away traveling at 72 kilometers per hour in the same direction. What is the perceived frequency at which the camera picks up the gunshot if the frequency of the gunshot is generally 800Hz?
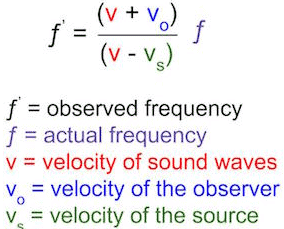
Answer Explanation: (D)
According to the Doppler equation, when velocity is added to the direction in which sound is traveling, it will increase the relative frequency
at which that sound is heard. The doppler equation is listed above:
This equation leads to a fairly simple mathematical problem. Velocity of sound waves is 343 m/s and should be known, although it will usually
be given. Thus, the answer is easily arrived at. To check work, make sure that if velocity is being added in the positive direction, that the frequency
increases.
The ability of the eukaryotic cell to compress coding sections when they are not being expressed is one of the numerous reasons why it can store so much information in its DNA. Which of the following processes will usually result in a decrease in gene expression when acting on DNA?
Answer Explanation: (A)
This is a straightforward question that assesses knowledge of modification of DNA segments. Although in the real world these methods are
not exclusive to increasing or decreasing activity, for MCAT testing purposes, histone acetyltransferase (HAT) frees DNA from histone proteins
for expression. Histone deacetyltransferase (HDAC) binds DNA more tightly to histone, creating more heterochromatin, which is not expressed.
Methylation is an epigenetic modification that generally trends toward silencing gene expression.
What is the most precise description of the reaction displayed?
Ca(OH)2 (aq) + H2SO4 (aq) → CaSO4 (aq) + H2O (l)
The correct answer is: A
This reaction is a classic example of a neutralization reaction, in which an acid and a base react to form a salt and, usually, water.
Although this reaction also fits the criteria for a double- displacement reaction, choice (D), in which two molecules essentially
exchange ions with each other, neutralization is a more specific description of the process.
The kinetics of SN1 reactions are first-order because:
The correct answer is: D
An SN1 reaction is a first-order nucleophilic substitution reaction. It is called first-order because the rate-limiting step involves only one
molecule. Choice (B) is true, but does not explain why SN1 reactions have first-order kinetics; the rate- limiting step of an SN2 reaction
is also the first (and only) step of that reaction, but SN2 reactions have second-order kinetics, not first-order. Choice (A) is a true statement
as well, but again does not explain why the reaction is first- order. Finally, choice (C) is incorrect because it is the rate- limiting step, not the
reaction overall, that involves only one molecule.
A man walks east for 30 meters and then north for 40 meters. What is the difference between his displacement and his traveled distance?
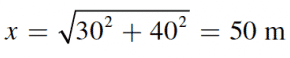
Using the Pythagorean theorem, calculate the magnitude of the man’s displacement:
His total distance traveled is equal to 30 + 40 = 70 m. Therefore, the difference between these two is 20 m.
A 30 kg female sits on the fulcrum of a seesaw at a distance of 2 meters. If her father weighs 90 kg, where should he sit to keep the seesaw balanced?
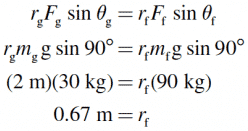
In order for the seesaw to be balanced, the torque due to the girl (τg ) must be exactly counteracted by the torque due to her father (τf ).
In other words, the magnitudes of these torques must be equal (τg = τf ):
Because r represents the distance of each person from the fulcrum, the father must sit 67 cm from the fulcrum.
A redox titration differs from other titrations in that it involves:
The correct answer is D:
Explanation:
This is the only answer choice that is unique to a redox titration. The other choices are common to various types of titrations.